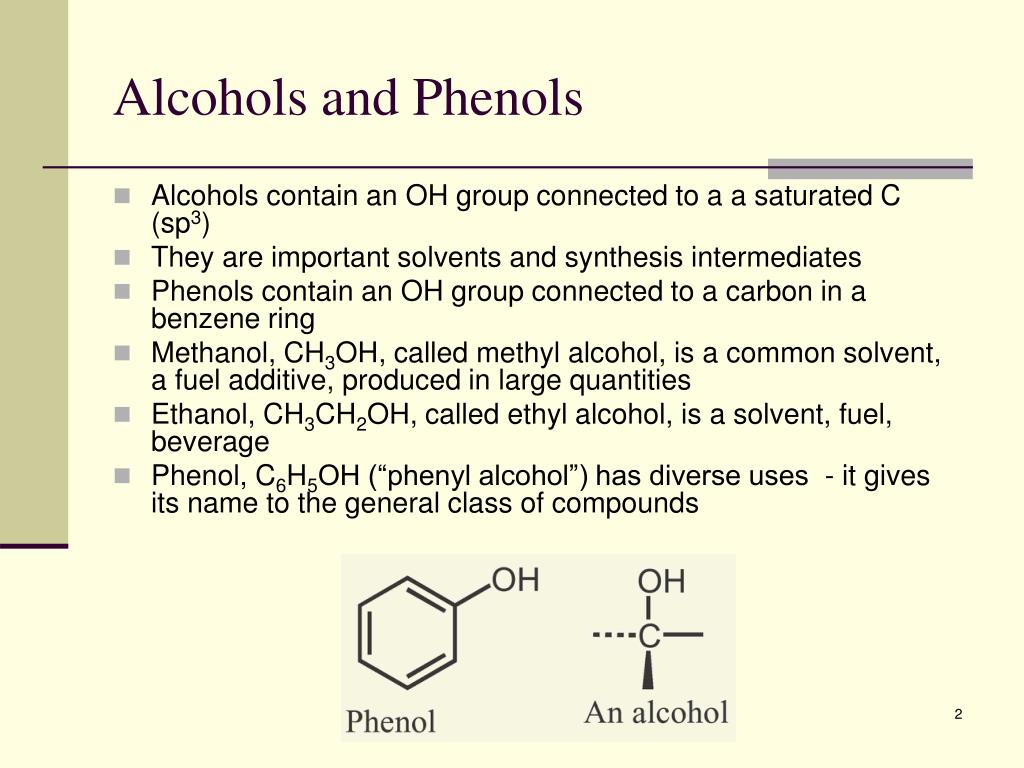
How To Distinguish Between Alcohol And Phenol . They react with aqueous sodium hydroxide (naoh) to form salts. discuss the factors that are believed to determine the acidity of alcohols and phenols. Substituent groups on an aromatic ring. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. difference between alcohol and phenol; phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Delocalization of the negative charge over the ortho and para positions of. phenols differ from alcohols in that they are slightly acidic in water. List a given series of alcohols or phenols in.
from www.slideserve.com
phenols differ from alcohols in that they are slightly acidic in water. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. They react with aqueous sodium hydroxide (naoh) to form salts. difference between alcohol and phenol; although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. Substituent groups on an aromatic ring. discuss the factors that are believed to determine the acidity of alcohols and phenols. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. List a given series of alcohols or phenols in. Delocalization of the negative charge over the ortho and para positions of.
PPT Alcohols and Phenols PowerPoint Presentation, free download ID
How To Distinguish Between Alcohol And Phenol discuss the factors that are believed to determine the acidity of alcohols and phenols. They react with aqueous sodium hydroxide (naoh) to form salts. difference between alcohol and phenol; phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. List a given series of alcohols or phenols in. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. Delocalization of the negative charge over the ortho and para positions of. Substituent groups on an aromatic ring. discuss the factors that are believed to determine the acidity of alcohols and phenols. phenols differ from alcohols in that they are slightly acidic in water. although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol.
From pediaa.com
Difference Between Phenol and Phenyl Definition, Structure, Properties How To Distinguish Between Alcohol And Phenol List a given series of alcohols or phenols in. They react with aqueous sodium hydroxide (naoh) to form salts. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. phenols differ from alcohols in that they are slightly acidic in water. Substituent groups on an aromatic ring. although. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols How To Distinguish Between Alcohol And Phenol discuss the factors that are believed to determine the acidity of alcohols and phenols. phenols differ from alcohols in that they are slightly acidic in water. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. although phenols are more acidic compared to alcohols, we can say that phenols are considered as. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols How To Distinguish Between Alcohol And Phenol difference between alcohol and phenol; discuss the factors that are believed to determine the acidity of alcohols and phenols. They react with aqueous sodium hydroxide (naoh) to form salts. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. phenols differ from alcohols in that they are slightly acidic in water. . How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT Structures of Alcohols, Phenols, Thiols and Ethers PowerPoint How To Distinguish Between Alcohol And Phenol phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. difference between alcohol and phenol; Substituent groups on an aromatic ring. phenols differ from alcohols in that they are slightly acidic in water.. How To Distinguish Between Alcohol And Phenol.
From www.differencebetween.net
Difference Between Alcohol and Phenol Difference Between How To Distinguish Between Alcohol And Phenol The alcohols are a class of organic compounds that hold at least one hydroxyl functional. Substituent groups on an aromatic ring. They react with aqueous sodium hydroxide (naoh) to form salts. although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. List a given series of alcohols or phenols. How To Distinguish Between Alcohol And Phenol.
From www.youtube.com
Distinguish Between Alcohol And Phenol Chemical Test Of Phenol How To Distinguish Between Alcohol And Phenol although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. discuss the factors that are believed to determine the acidity of alcohols and phenols. Substituent groups on an. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID How To Distinguish Between Alcohol And Phenol Delocalization of the negative charge over the ortho and para positions of. difference between alcohol and phenol; phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. They react with aqueous sodium hydroxide (naoh) to form salts. List a given series of alcohols or phenols in. The alcohols are. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT ALCOHOLS and PHENOLS PowerPoint Presentation, free download ID How To Distinguish Between Alcohol And Phenol phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (naoh) to form salts. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. List a given series of alcohols or phenols in. The alcohols are a class of organic compounds. How To Distinguish Between Alcohol And Phenol.
From www.youtube.com
Difference Between Alcohol And Phenol?Class Series YouTube How To Distinguish Between Alcohol And Phenol They react with aqueous sodium hydroxide (naoh) to form salts. Substituent groups on an aromatic ring. although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. phenols differ from alcohols in that they are slightly acidic in water. Delocalization of the negative charge over the ortho and para. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation ID233852 How To Distinguish Between Alcohol And Phenol although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. List a given series of alcohols or phenols in. difference between alcohol and phenol; Delocalization of the negative charge over the ortho and para positions of. The alcohols are a class of organic compounds that hold at least. How To Distinguish Between Alcohol And Phenol.
From thecontentauthority.com
Alcohols vs Phenols Differences And Uses For Each One How To Distinguish Between Alcohol And Phenol phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (naoh) to form salts. Substituent groups on an aromatic ring. The alcohols are a class of organic compounds that hold at. How To Distinguish Between Alcohol And Phenol.
From askfilo.com
related to phenol. Ans. Differences between Phenol and Alcohol (M.P. 20.. How To Distinguish Between Alcohol And Phenol phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. List a given series of alcohols or phenols in. discuss the factors that are believed to determine the acidity of alcohols and phenols. The alcohols are a class of organic compounds that hold at least one hydroxyl functional. They. How To Distinguish Between Alcohol And Phenol.
From www.youtube.com
Alcohol Phenol Class 12 / Distinguish test / primary secondary tertiary How To Distinguish Between Alcohol And Phenol The alcohols are a class of organic compounds that hold at least one hydroxyl functional. List a given series of alcohols or phenols in. Delocalization of the negative charge over the ortho and para positions of. phenols differ from alcohols in that they are slightly acidic in water. Substituent groups on an aromatic ring. They react with aqueous sodium. How To Distinguish Between Alcohol And Phenol.
From chem.libretexts.org
1.2 Functional groups and organic nomenclature Chemistry LibreTexts How To Distinguish Between Alcohol And Phenol The alcohols are a class of organic compounds that hold at least one hydroxyl functional. Delocalization of the negative charge over the ortho and para positions of. Substituent groups on an aromatic ring. phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (naoh) to form salts. phenols are more. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols How To Distinguish Between Alcohol And Phenol phenols differ from alcohols in that they are slightly acidic in water. discuss the factors that are believed to determine the acidity of alcohols and phenols. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. although phenols are more acidic compared to alcohols, we can say. How To Distinguish Between Alcohol And Phenol.
From studylib.net
Alcohols and Phenols How To Distinguish Between Alcohol And Phenol discuss the factors that are believed to determine the acidity of alcohols and phenols. They react with aqueous sodium hydroxide (naoh) to form salts. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Substituent groups on an aromatic ring. although phenols are more acidic compared to alcohols,. How To Distinguish Between Alcohol And Phenol.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols How To Distinguish Between Alcohol And Phenol The alcohols are a class of organic compounds that hold at least one hydroxyl functional. Substituent groups on an aromatic ring. They react with aqueous sodium hydroxide (naoh) to form salts. phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. List a given series of alcohols or phenols in.. How To Distinguish Between Alcohol And Phenol.
From www.youtube.com
Distinction between Alcohol and Phenol/ Uses of Phenol / AJT Chemistry How To Distinguish Between Alcohol And Phenol phenols differ from alcohols in that they are slightly acidic in water. Substituent groups on an aromatic ring. although phenols are more acidic compared to alcohols, we can say that phenols are considered as a subset of alcohol. List a given series of alcohols or phenols in. discuss the factors that are believed to determine the acidity. How To Distinguish Between Alcohol And Phenol.